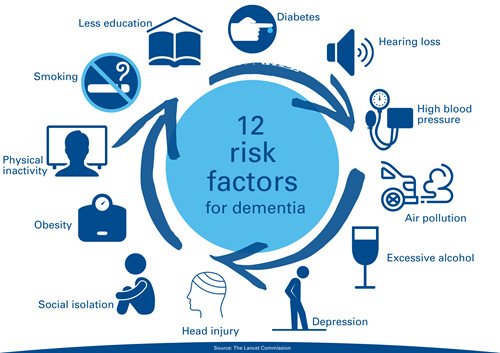
Decision On Crisis Use Listing Of Covaxin
Decision On Crisis Use Listing Of Covaxin. The World Health Organization (WHO) is probably going to take a choice on including Bharat Biotech’s COVID-19 immunization Covaxin in the crisis use list (EUL) inside four to about a month and a half, Soumya Swaminathan, the worldwide wellbeing body’s main researcher has said.
Talking at an online class coordinated by the Center for Science and Environment (CSE) on Friday, Ms Swaminathan said the WHO is looking into Covaxin as its producer Bharat Biotech is currently transferring its whole information on the wellbeing body’s gateway.
As indicated by WHO rules, EUL is a method to smooth out the cycle by which new or unlicensed items can be utilized during general wellbeing crises.
“There is an interaction to be followed for EUL and pre-capability of antibodies under which an organization needs to finish stage 3 preliminaries and present the entire information to the administrative branch of WHO which is analyzed by a specialist warning gathering,” Ms Swaminathan said.
“The culmination of the information, which incorporates security and adequacy and furthermore the assembling quality, standard is given. Along these lines, I expect that Bharat Biotech has effectively submitted information and in four to about a month and a half there will be a choice on its incorporation,” Ms Swaminathan added.
As of now, the WHO has endorsed immunizations by Pfizer/BioNTech, Astrazeneca-SK Bio/Serum Institute of India, AstraZeneca EU, Janssen, Moderna and Sinopharm for crisis use.

“We as of now have six immunizations endorsed with EUL and have proposals from our Strategic Advisory Group of Experts (SAGE). We keep on taking a gander at Covaxin. Bharat Biotech has now begun transferring their information on our entryway and that is the following immunization that will be explored by our specialists panel,” the main researcher said.
She additionally referenced the WHO Research and Development Blueprint ready in 2016, soon after the Ebola episode, in which an examination guide for infections with pandemic potential was spread out.
“I need to specify the Research and Development (R&D) Blueprint. I think we need to consider how we can improve in the future as far as advancement of antibodies as well as medications, diagnostics and guaranteeing evenhanded access. This diagram was created after the Ebola flare-up and basically it’s anything but an examination guide for infections which have pandemic potential,” she said.
“In this way, when the guide was created in 2016, it referenced “Microorganism X” in it which showed that we were expecting a pandemic, which is currently COVID-19,” she added.
Swaminathan additionally said the guide basically spread out the means as far as creating objective item profiles – like guidelines for antibodies, diagnostics administrative norms, preliminary plans, and preliminary test systems.
“This pre-thinking helped on the grounds that WHO had the option to unite researchers, specialists, scholastics and organizations toward the start of last year to foster an exploration guide for COVID,” she said.
As of now, there are 105 competitor immunizations in clinical assessment out of which 27 are in stage three or four, she said.
There are another 184 applicant immunizations in preclinical assessment. A large portion of the immunizations are intended for a two-portion plan, she added.
The WHO boss researcher additionally said the Delta variation of the Covid is truly contagious.
“Two complete portions are needed for assurance against the Delta variation however you can in any case get the contamination and can communicate it. This is the reason concealing and different safeguards are imperative to proceed,” she said.
Discussing a few organizations pushing on the need to foster a promoter portion after two antibody shots dosages to ensure individuals, Swaminathan said, now there is no information to show if a sponsor portion is required.
“Science is advancing. Now we don’t have information to demonstrate that everybody will require a supporter and is it will be following one little while a long time. Yet, information from follow up investigations of immunized individuals are extremely promising and is showing that safe reactions are enduring up to 8, 10 or even a year,” she said.
“A couple of studies that have taken a gander at giving supporter portion following a half year have shown that it can expand the neutralizer levels so high that they can secure against all variations. What we know is you need an undeniable degree of antibodies, regardless of whether it’s through a promoter or the main course. We need more investigations and see which immunizations will require a sponsor and when. It very well may be conceivable that a mix of two unique antibodies is managed in future yet these are altogether being investigated through research thus we need to pause,” she said.